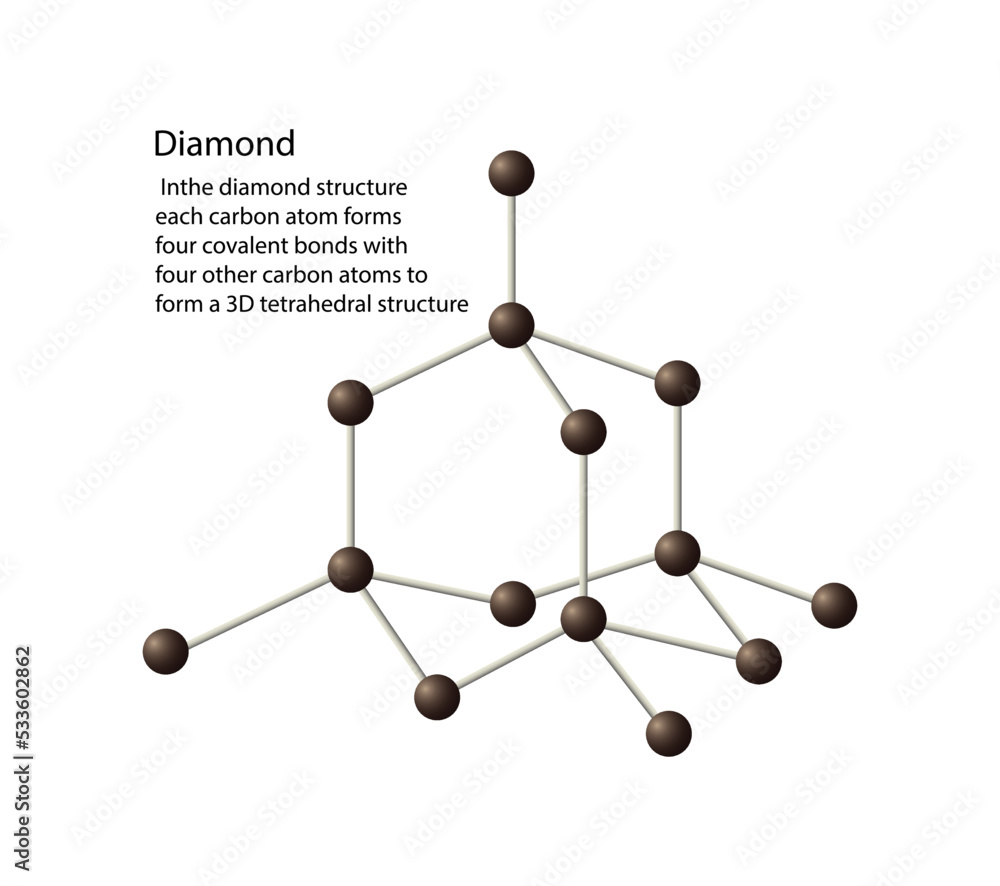
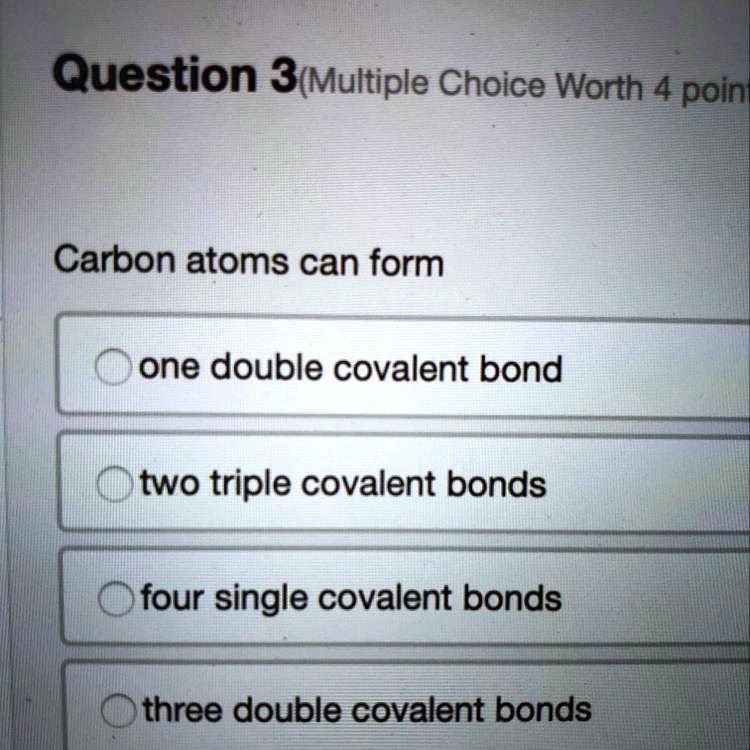
A Carbon Atom Can Form Up To Four Covalent Bonds - A carbon atom can form up to four covalent bonds. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Well, carbon can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its. Carbon can form four covalent bonds to create an organic molecule.
A carbon atom can form up to four covalent bonds. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon can form four covalent bonds to create an organic molecule. Carbon has four valence electrons, so it can achieve a full outer energy level by. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Well, carbon can form up to four covalent bonds.
Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its. Carbon can form four covalent bonds to create an organic molecule. Well, carbon can form up to four covalent bonds. A carbon atom can form up to four covalent bonds.
__TOP__ How Many Covalent Bonds Can Chlorine Form
A carbon atom can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. Carbon can form four covalent bonds to create an organic molecule. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Well, carbon can form up to four covalent bonds.
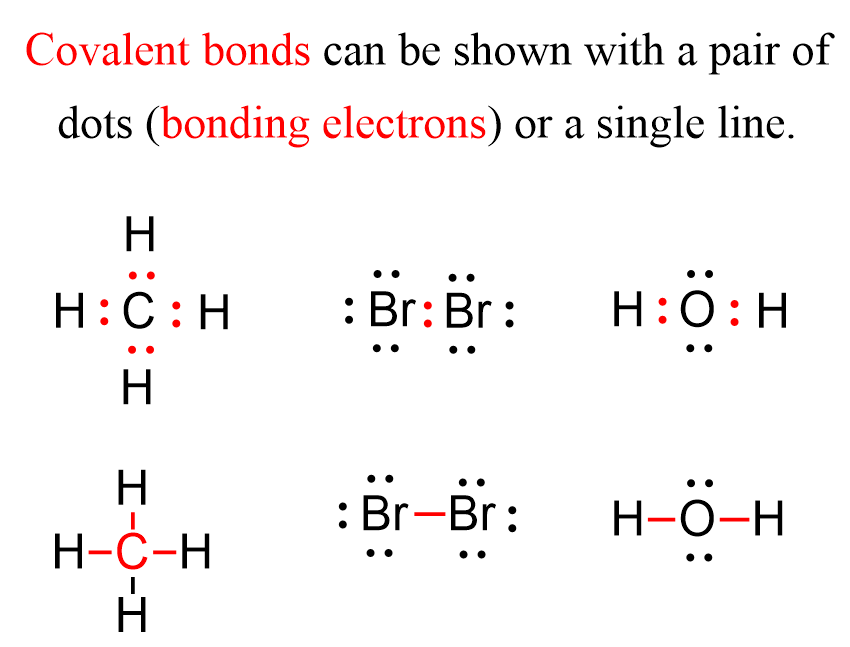
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon can form four covalent bonds to create an organic molecule. Carbon can form four covalent bonds to create an organic molecule. The _____ functional group(s) is/are found within amino acids,.
Covalent bonding tecscience
Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon can form four covalent bonds to create an organic molecule. The _____ functional group(s) is/are found within amino acids, while carbohydrates. Carbon has four valence electrons, so it can achieve a full outer energy level by. Well, carbon can form up to four covalent bonds.
Vetor de illustration of chemistry, The diamond structure each carbon
The _____ functional group(s) is/are found within amino acids, while carbohydrates. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. A carbon atom can form up to four covalent bonds. A carbon atom has six electrons in its. Well, carbon can form up to four covalent bonds.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
Carbon can form four covalent bonds to create an organic molecule. The _____ functional group(s) is/are found within amino acids, while carbohydrates. A carbon atom has six electrons in its. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. A carbon atom can form up to four covalent bonds.
Covalent Bond Chemistry Steps
Well, carbon can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its. The _____ functional group(s) is/are found within amino acids, while carbohydrates.
SOLVED Carbon atoms can form... A) one double covalent bond B) two
Study with quizlet and memorize flashcards containing terms like a carbon atom can form. A carbon atom can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. A carbon atom has six electrons in its. Well, carbon can form up to four covalent bonds.
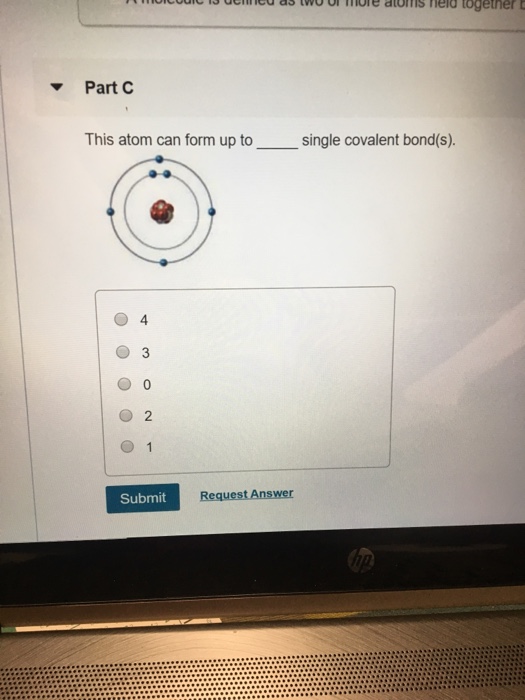
Solved Part C This atom can form up to single covalent
A carbon atom can form up to four covalent bonds. Carbon can form four covalent bonds to create an organic molecule. Carbon has four valence electrons, so it can achieve a full outer energy level by. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. A carbon atom has six electrons in its.
SOLVED Mark the following statements about carbon as true or false A
Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Carbon can form four covalent bonds to create an organic molecule. Well, carbon can form up to four covalent bonds. Carbon can form four covalent bonds to create an organic molecule. A carbon atom has six electrons in its.
CH104 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
A carbon atom can form up to four covalent bonds. Carbon has four valence electrons, so it can achieve a full outer energy level by. Well, carbon can form up to four covalent bonds. Carbon can form four covalent bonds to create an organic molecule. The _____ functional group(s) is/are found within amino acids, while carbohydrates.
Carbon Can Form Four Covalent Bonds To Create An Organic Molecule.
A carbon atom has six electrons in its. Study with quizlet and memorize flashcards containing terms like a carbon atom can form. Well, carbon can form up to four covalent bonds. The _____ functional group(s) is/are found within amino acids, while carbohydrates.
A Carbon Atom Can Form Up To Four Covalent Bonds.
Carbon has four valence electrons, so it can achieve a full outer energy level by. Carbon can form four covalent bonds to create an organic molecule.