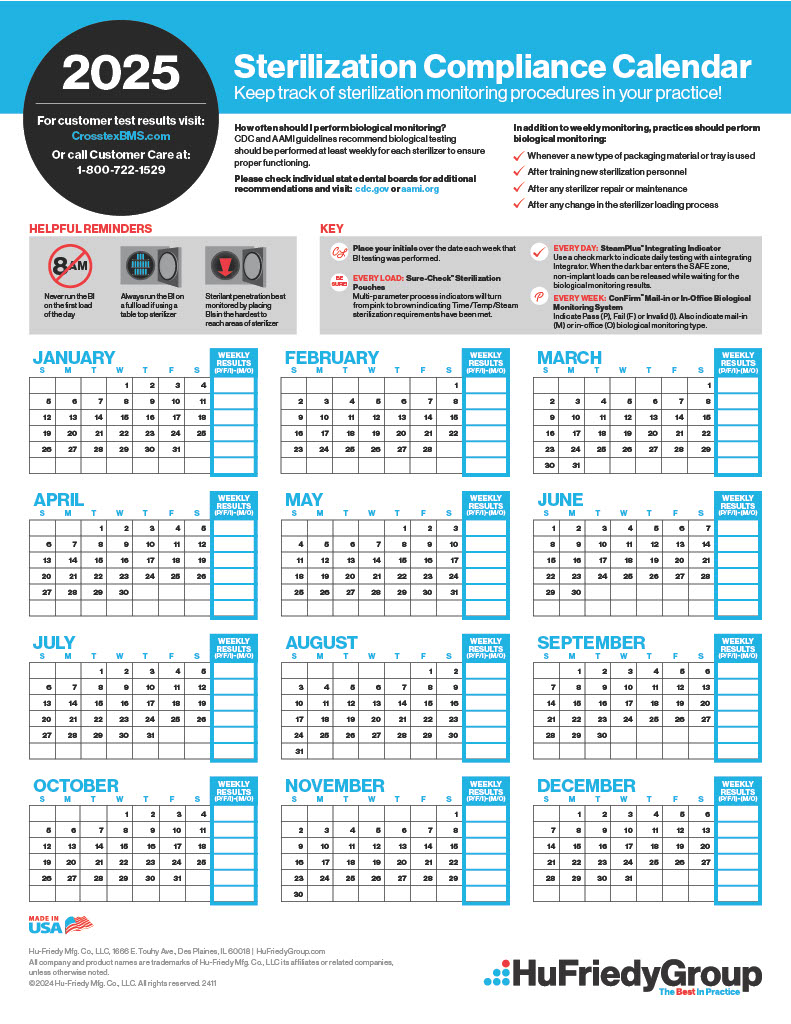
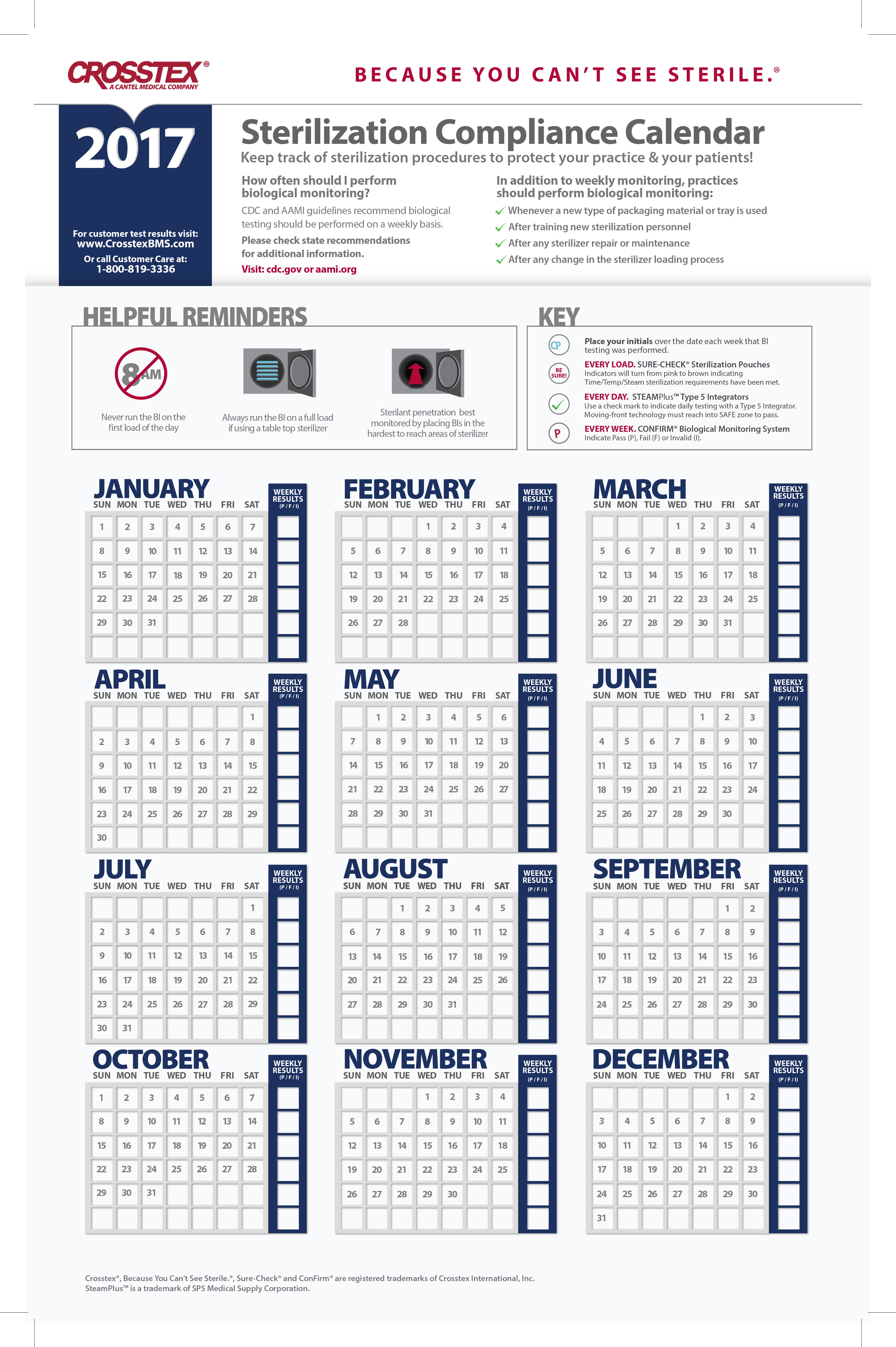
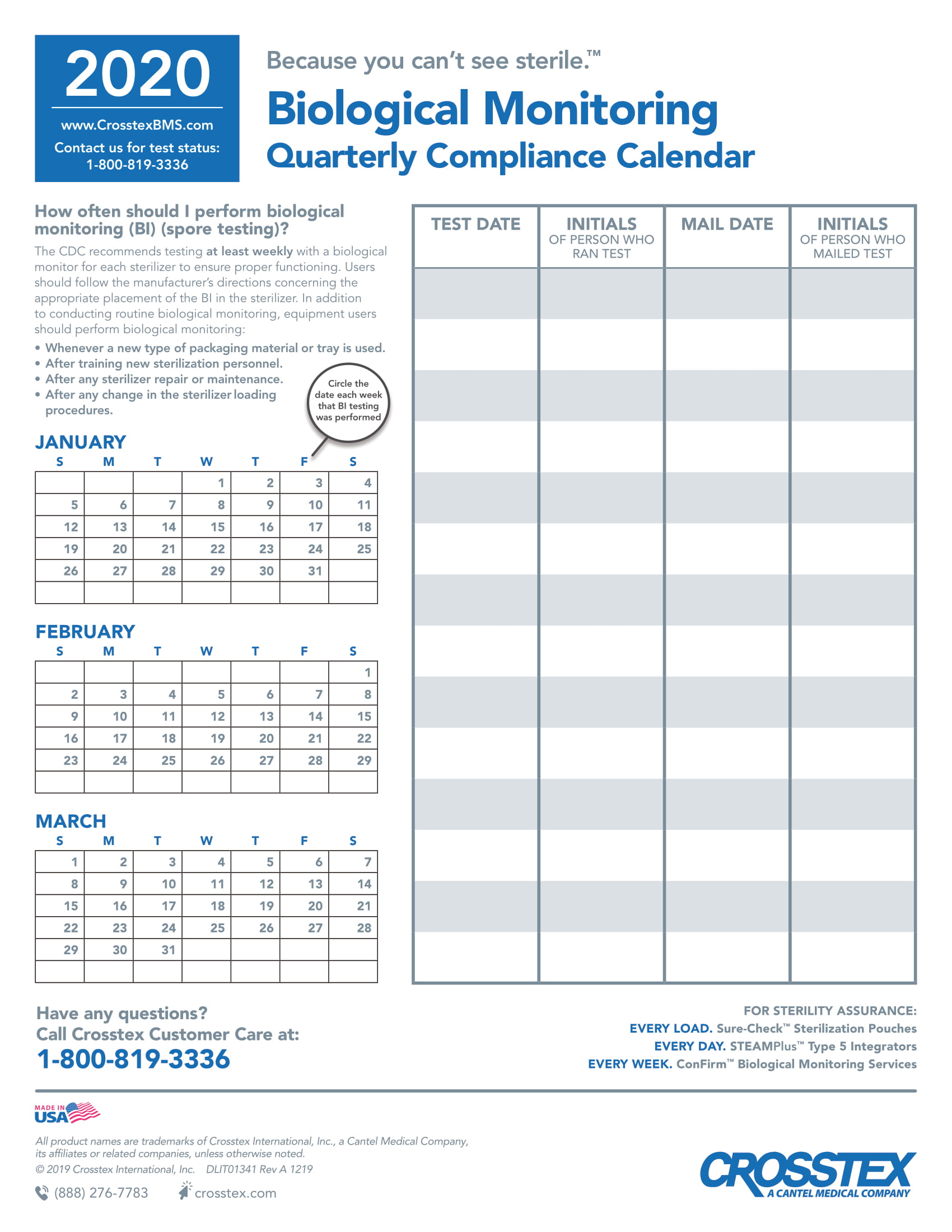
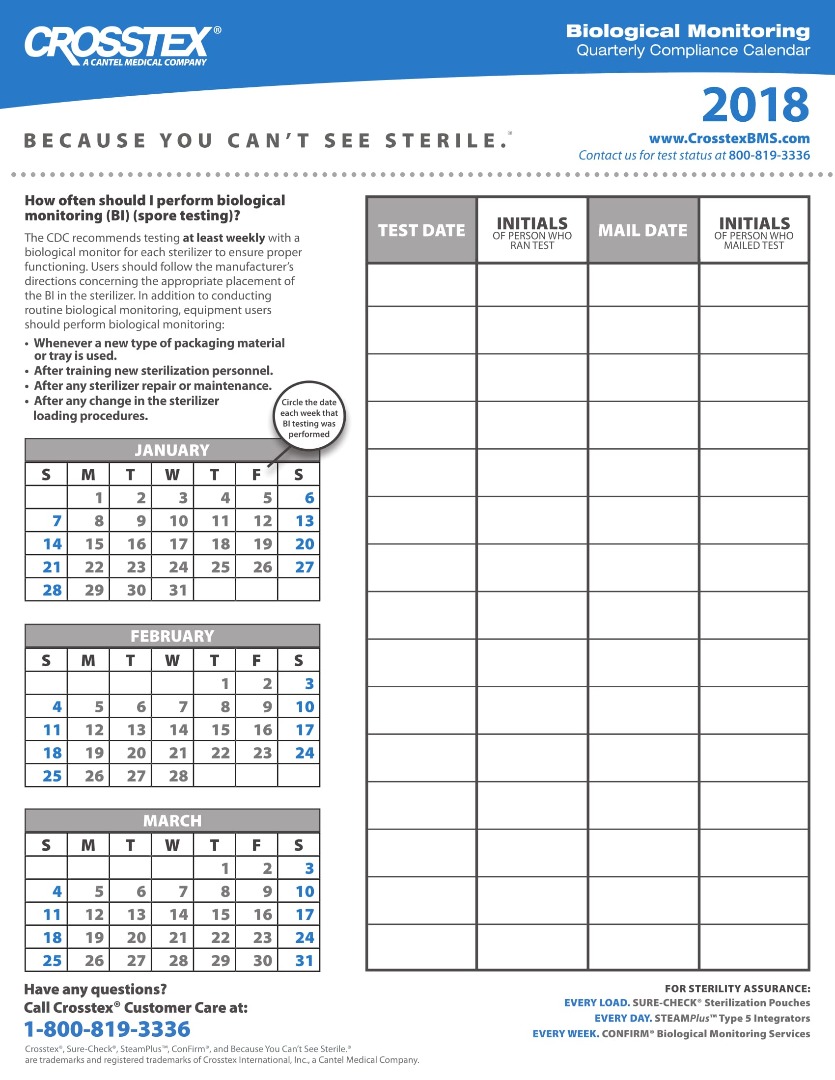
Sterilization Compliance Calendar 2026 - 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. Regulatory compliance watch makes available our 2025 compliance calendar to our members. The environmental protection agency (epa) has set an october 5, 2026, deadline for sterilization facilities to comply with. Download it to your current calendar. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial.
The environmental protection agency (epa) has set an october 5, 2026, deadline for sterilization facilities to comply with. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. Download it to your current calendar. Regulatory compliance watch makes available our 2025 compliance calendar to our members. As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements.
Regulatory compliance watch makes available our 2025 compliance calendar to our members. 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. The environmental protection agency (epa) has set an october 5, 2026, deadline for sterilization facilities to comply with. Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. Download it to your current calendar. As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial.
Tools Crosstex Biological Monitoring Service Provider
Regulatory compliance watch makes available our 2025 compliance calendar to our members. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory.
Tools Crosstex Biological Monitoring Service Provider
Download it to your current calendar. Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. The.
Sterilization Compliance Calendar 2025 Karie Leanna
Regulatory compliance watch makes available our 2025 compliance calendar to our members. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. Download it to your current calendar. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. The environmental protection agency (epa) has set an october 5, 2026,.
Crosstex Sterilization Compliance Calendar Bobby Nicoli
Download it to your current calendar. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. Regulatory compliance watch makes available our 2025 compliance calendar to our members. Cdc and aami guidelines recommend biological testing should.
Tools Crosstex Biological Monitoring Service Provider
8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. Regulatory compliance watch makes available our 2025 compliance calendar to our members. Download it to your current calendar. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. The environmental protection agency (epa) has set an october.
Sterilization Compliance Calendar 2025 Karie Leanna
8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. Cmi is pleased to provide a compliance.
Sterilization Standards Update Strategies for Compliance PPT
8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. Regulatory compliance watch makes available our 2025 compliance calendar to our members. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting.
Sterilization Compliance Calendar 2025 Karie Leanna
Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. Regulatory compliance watch makes available our 2025 compliance calendar to our members. As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. 8.5x11 biological monitoring quarterly compliance calendar keep.
Tsd Calendar 2025 2026 Tx Gus Honoria
Cdc and aami guidelines recommend biological testing should be performed at least weekly for each sterilizer to ensure proper functioning. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial. The environmental protection agency (epa) has set an october.
Tools Crosstex Biological Monitoring Service Provider
Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients. As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. Regulatory compliance watch makes available.
The Environmental Protection Agency (Epa) Has Set An October 5, 2026, Deadline For Sterilization Facilities To Comply With.
Download it to your current calendar. Regulatory compliance watch makes available our 2025 compliance calendar to our members. Cmi is pleased to provide a compliance calendar/checklist of upcoming federal regulatory deadlines and reporting requirements. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial.
Cdc And Aami Guidelines Recommend Biological Testing Should Be Performed At Least Weekly For Each Sterilizer To Ensure Proper Functioning.
As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or. 8.5x11 biological monitoring quarterly compliance calendar keep track of sterilization procedures to protect your practice & your patients.